Is Combustibility A Chemical Property
Introduction
The application of big-format lithium-ion batteries (LIBs) with high energy density in electrical vehicles requires a loftier level of battery prophylactic1,two,3,4,5,6,seven,8,9, as the fires and explosions of batteries that take place during thermal runaway pose serious threats to the lives of passengers and property1,x,xi,12. Thermal runaway is driven by a chain of exothermic reactions that spontaneously increment the temperature of lithium-ion batteries. As a result, severe redox exothermic reactions are likely triggered at relatively high temperatures, generating a tremendous amount of heat and leading to uncontrollable rise in temperature11,12,13,14,15,16,17,xviii,19. Thus, the removal or reduction of the main exothermic reactions during the evolution of thermal runaway is essential to guarantee the safety of lithium-ion batteries.
Flammable carbonate-based electrolytes accept been widely used in commercial LIBs, and they are considered to be responsible for thermal runaway2,3,20, which is reasonable, as they are among the main fuels that increase vigorous combustion. Thus, non-flammable electrolytes are considered and thought to improve battery safety. Concentrated electrolytes and localized or aqueous concentrated electrolytes accept been receiving considerable attention in recent years, due to their high electrochemical performance, low volatility, and depression flammability20,21,22,23,24,25,26,27. Many fundamental studies revealed that the unique solution construction, where all the solvent molecules and even anions are involved in the solvation sheath, endow concentrated electrolytes with different backdrop in comparison with the same compositional merely traditional 1 1000 electrolytes. For case, in a battery, when the molar ratio of lithium bisimide (fluorosulfonyl) (LiN(So2F)2, LiFSI) to the solvent is 1:ane.9, the predominant contact ion pairs (CIPs, an anion analogous to one Li+) and aggregates (AGGs, an anion coordinating to two or more than Li+) in the solvated structures guarantee the stable cycling and ameliorate thermal properties of LIBs22,28,29. Meanwhile, burn down retardant solvents, such as trimethyl phosphate (TMP), triethyl phosphate (TEP), and trisphosphate (trifluoroethyl) (TFEP), can exist employed to develop non-flammable electrolytes due to the changes in the interface reactions that are led by the unique solvation structure of concentrated electrolytes20,24,thirty. However, although the high thermal stability of concentrated electrolytes, which enhances battery safety as implied past the researchers, has been widely proven by ignition tests and thermogravimetric analyses (TGA)xx,24,29. No measurements using a practical bombardment have been reported to directly verify the improved safety of LIBs with advanced concentrated electrolytes. It has been reported that the highly energetic thermal delinquent may release just a minor corporeality of rut in flaming combustion and vice versa31,32.
Thus, a direct measurement of the safety of batteries with concentrated electrolytes is necessary to clarify the truth. In addition, the proposed mechanism will deepen our understanding of thermal delinquent. Generally, it is understandable that stopping the triggered redox exothermic reactions can assistance reduce the destructiveness of battery thermal runaway, which can be achieved by, for instance, replacing the layered cathode fabric with LiFePOiv. Moreover, avoiding the initial triggers or shutting downwardly the reaction chain is very meaningful for safe control. For most of the bombardment chemistries with layered oxide cathodes and graphite anodes, such as graphite|LiNi0.3Co0.iiiMn0.iiiO2 (Gr|NMC333) batteries, it was confirmed that the heat generated before 250 °C is dominated by the reactions between the anode and the electrolyte, which is believed to extremely increment the bombardment'due south temperature to a level that initiates the concluding thermal runaway reactions7,33,34. Besides, the chemical crosstalk in graphite|LiNi0.5Mn0.3 Co0.twoO2 (Gr|NMC532) batteries was proven to trigger thermal runaway without internal short circuits (ISC)12. In add-on, the lithiated anode consumes the highly oxidative gases released during the cathode phase transition, thus producing tremendous estrus that brings the battery to the thermal runaway point12. In this case, the electrolyte is a dispensable ingredient for thermal delinquent. Roughly, the exothermic reactions inside the battery tin can be divided into three groups: the reactions between the anode-electrolyte (AnEly), the reactions between the cathode-electrolyte (CaEly), and the reactions between the cathode-anode (CaAn)11. Among these reactions, the AnEly reactions contribute to the initial estrus aggregating, and the CaEly and CaAn reactions both outcome in drastic combustion, but they demand a considerable loftier temperature to offsetxi,12,33. Considering that non-flammability is an essential result of the CaEly reactions, predicting the safety of a charged battery is bereft.
In this work, we use accelerated rate calorimetry (ARC), differential scanning calorimeter (DSC) coupled with thermal gravimetric analysis (TGA), and mass spectrometry (DSC-TG-MS) experiments to evaluate the safety performance of graphite|LiNi0.eightCo0.aneMn0.1O2 (Gr|NMC811) and Gr|NMC532 batteries with concentrated electrolytes. The thermal runaway mechanism at both the jail cell and cloth level is systematically investigated. The most promising concentrated electrolytes, LiFSI/DMC (1:ane.9 by molar) and LiFSI/TMP (1:1.nine by molar), are selected in this written report. The results bear witness that the non-flammable concentrated electrolytes could not prevent LIBs from thermal runaway. Specifically, the charged anode reacts with LiFSI and releases a considerable corporeality of heat that triggered thermal delinquent.
Results
Cycling and thermal properties of the electrolytes
Figure 1a shows that the 0.93Ah Gr|NMC811 pouch cell with the concentrated LiFSI/DMC electrolyte delivered stable charge-discharge capacities for over 300 cycles at C/three. The average coulombic efficiency was 96.6% and capacity retentivity was 94.five% (Fig. 1b), indicated the suppressed Al dissolution and stable SEI on the graphite during cycling20,24,29. The cell with conventional ane Chiliad LiPFvi/EC:EMC (3:7 past volume) electrolyte shows comparable electrochemical performances, the capacity retention after 300 cycles was 93.ix%. The LiFSI/TMP concentrated electrolyte in the pouch jail cell was also investigated for two cycles before safety evaluation, and it delivered a coulombic efficiency of 99.5% (Fig. 1a). For the Gr|NMC532 pouch cells, the charge and belch curves also demonstrated stable electrochemical performance with the concentrated electrolytes (Supplementary Fig. i and Supplementary Note 1).
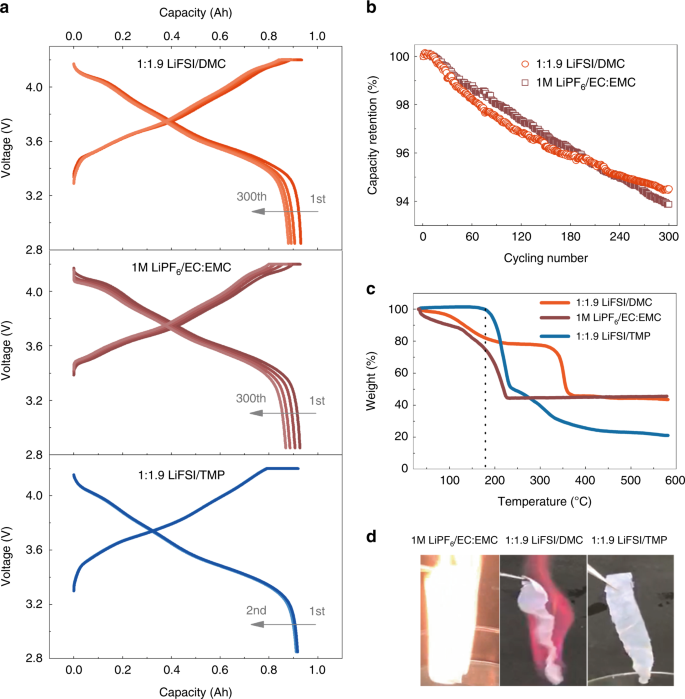
a Accuse and belch plots of the Gr|NMC811 battery with LiFSI/DMC (one:1.nine by molar), LiFSI/TMP (1:i.9 by molar), and conventional one Yard LiPFsix/EC:EMC (3:seven by volume). All batteries delivered a reversible chapters of 0.93 Ah, which was approximated to the design capacity of 0.95 Ah. b Cycling operation of Gr|NMC811 bombardment with LiFSI/DMC full-bodied electrolyte and i K LiPFhalf dozen/EC:EMC. c TGA curves showed the weight loss of the LiFSI/DMC and LiFSI/TMP full-bodied electrolytes and the one M LiPF6/EC:EMC electrolyte. d Flammability of the LiFSI/DMC and LiFSI/TMP concentrated electrolytes and the 1 M LiPF6/EC:EMC electrolyte. Ignition tests were performed using polyethylene separators, which were saturated with the electrolytes. A flame igniter produced a flame with a temperature of above 1400 °C. The photos displayed the moment when the electrolytes were burning with the most vigorous flame.
The TGA curves (encounter Fig. 1c) show that the weight loss of the LiFSI/TMP full-bodied electrolyte was only 0.7 wt% below 180 °C, which is considerably lower than that of the LiFSI/DMC electrolyte (18.2 wt%) and the diluted carbonate electrolyte (26.five wt%). These results also indicate that the flammability of the LiFSI/DMC concentrated electrolyte is lower than that of the dilute conventional electrolyte, as less solvent was used and the vitality of the DMC was significantly changed by the solvation construction. Then, Fig. 1d shows the photographs of the separators saturated with the electrolytes during the ignition test. In comparison with the conventional electrolyte, the concentrated electrolyte with the DMC was nevertheless flammable but with a mild flame, whereas the concentrated electrolyte with the self-extinguishing solvent of the TMP did non burn completely, which proves that the full-bodied LiFSI/TMP is not-combustible (see details in Supplementary Table 1 and Supplementary Note 2). According to the higher up thermal evaluation, full-bodied electrolytes display meliorate thermal stability and possibly lower flammability than dilute electrolytes, which is in agreement with the previous reports24,29,30.
Nonetheless, it was reported that the direct redox reactions between the charged cathode and anode are severe for bombardment chemistries of high energy density, which can cause thermal runaway even without electrolytes or ISC12,13. Thus, the evaluation of battery rubber based on the used electrolytes only is non sufficient, and the interaction between the electrolytes and the charged electrodes should be systematically taken into consideration.
Safety label of LiFSI/DMC in Gr|NMC batteries
The thermal runaway features of Gr|NMC batteries with full-bodied LiFSI/DMC and conventional 1 G LiPFhalf dozen/EC:EMC electrolytes are compared in Fig. ii. It is observed that all the batteries were brought to the betoken of thermal delinquent although the thermal stability of the full-bodied electrolyte was plain college. The three characteristic temperatures of {T 1, T two, T 3} were defined to describe the thermal behavior of the batteries with unlike electrolytesseven,11,13. In this report, in case of the concentrated and conventional electrolytes, T 1 was located at ~130 °C, and T 3 was between 650 °C and 730 °C, whereas T 2 exhibited a totally different value (Fig. 2). T 2 was defined every bit the trigger temperature of the thermal runaway. At and afterward this critical point, the battery temperature exponentially increased and could not exist shut downwards by any heat dissipation measures. Both the severe exothermic reactions and the ISC might be the underlying reasons. If T 2 is acquired by a unmarried chemical reaction or a group of chemic reactions, this or these reactions can be divers as the trigger reaction of thermal runaway. The tremendous heat generated at T two by the trigger reaction would immediately trigger a unmarried exothermic reaction or a group of new exothermic reactions between the battery components, causing a dramatic ascension (hundreds of degrees per 2nd) of battery temperature. Understanding the mechanisms behind T two is crucial for pattern of safer lithium-ion batteries.
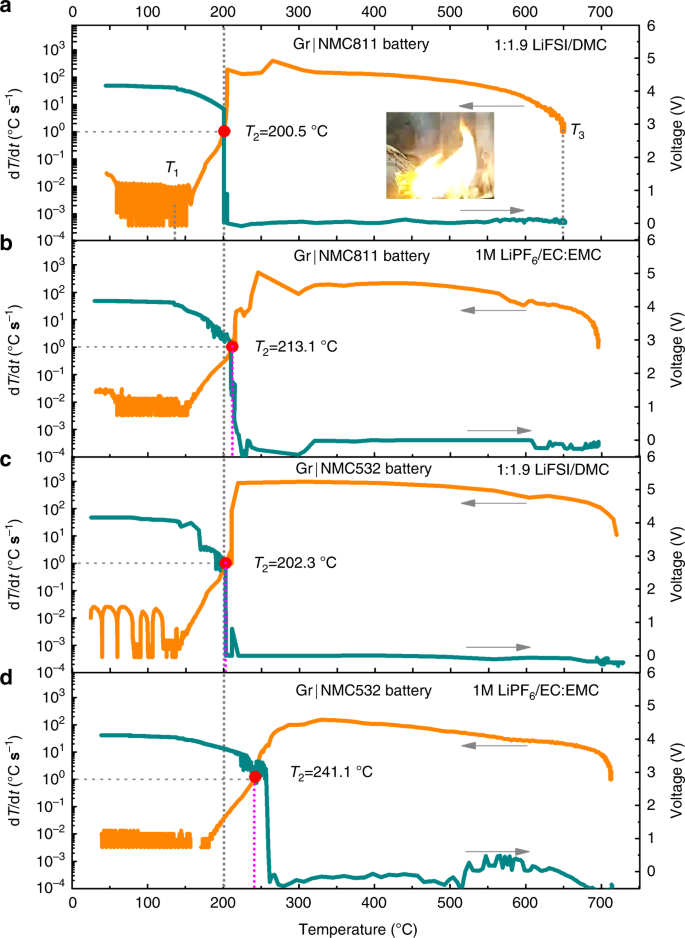
a Gr|NMC811 battery with the full-bodied LiFSI/DMC electrolyte. The inset shows the combustibility of bombardment in the lateral heating test. b Gr|NMC811 bombardment with the conventional ane K electrolyte. c Gr|NMC532 battery with the concentrated LiFSI/DMC electrolyte. d Gr|NMC532 battery with the conventional one Yard electrolyte. dT/dt-T curves of Gr|NMC811 and Gr|NMC532 batteries based on the ARC test, were plotted in logarithmic coordinates. T 1 was defined as the onset temperature of self-heating, which results from the onset of the chain reactions within the bombardment, leads to a spontaneous and continuous rise in temperature if the bombardment is kept under a poor estrus dissipation condition or an almost adiabatic condition. T 2 was divers as the trigger temperature of thermal runaway preset at the dT/dt of i °C s−1. T 3 was divers as the maximum temperature during the thermal runaway, which is a key parameter in the evaluation of the destructiveness of thermal runaway.
For the Gr|NMC811 battery with the LiFSI/DMC concentrated electrolyte (Fig. 2a), T 2 was located at 200.5 °C. A fall in the OCV took place at T ii, which coincides with the sharp temperature rise. Notwithstanding, T two of the cell with the conventional electrolyte reached 213.1 °C and simultaneously with OCV falling (come across Fig. 2b), which is 12.six °C higher than that of the case with the concentrated electrolyte. T 2 and OCV displayed the repeatable characters (Supplementary Fig. 2), and OCV did not autumn until ~213.1/214.eight °C, suggesting that the separators in the cells had the potential to stand 213.one °C/214.eight °C or even college temperature without ISC. Additionally, the heat generated by ISC was evaluated based on the internal resistance of battery around T 2. ISC just can contributed a (dT/dt)ISC of 0.06 °C s−one, much lower than one °C south−1 at T two (See details in Supplementary Annotation three). Thus, it concludes that, for Gr|NMC811 batteries with the concentrated electrolyte, the exothermic process that leads to T ii is caused by internal reactions and not by ISC. A large amount of heat was released in the bombardment. Every bit a outcome, the loss of the integrity of the separator or the battery bang-up accompanied with the vigorous exothermic reactions, would lead to a sharp drop in the voltageane,12. Later T ii, the battery temperature sharply increased to the maximum temperature (T 3 = 652.two °C) in 15.4 south. The maximum dT/dt during the thermal runaway was 401.2 °C s−i. Meanwhile, the vehement flame was observed in the lateral heating test (the inset in Fig. 2a), and the overall process is shown in Supplementary Movie 1, indicating that the battery with the concentrated LiFSI/DMC electrolyte was combustible during the thermal runaway even though the electrolyte demonstrated depression flammability.
The thermal features of the Gr|NMC532 bombardment with the concentrated LiFSI/DMC electrolyte were likewise examined (see Fig. 2c). The chemical reaction was also proved to exist the trigger for thermal runaway (meet details in Supplementary Note iii). T ii was found to exist 202.3 °C, which is shut to that of the Gr|NMC811 battery with the concentrated LiFSI/DMC electrolyte. However, the Gr|NMC532 battery with the conventional electrolyte still showed T 2 at a higher temperature (241.ane °C, see Fig. 2nd). It was reported that the crosstalk between the NMC532 cathode and anode was when the reactions happened at T 2 12. At or afterwards T 2, the nearly exothermic reactions, where the cathode acted as the main reactant, were initiated. So, it was understandable that Gr|NMC811 batteries always exhibited a lower T two than Gr|NMC532 batteries when with the same electrolyte35.
Information technology was interesting that the value of T 2 in the Gr|NMC811 battery was very shut to that of T 2 in the Gr|NMC532 battery when the aforementioned concentrated LiFSI/DMC electrolyte was used in both of them, and both values were lower than that of the T 2 in the battery with the conventional electrolyte. These phenomena indicated that the full-bodied LiFSI/DMC electrolyte could not heighten the intrinsic battery safe, even though the full-bodied electrolyte was more than thermally stable than the conventional electrolyte. So, the quite like T 2 of the NMC811 and NMC532 batteries with the LiFSI/DMC concentrated electrolyte point that similar chemical reactions occurred at ~200 °C and that these reactions brought both batteries to the betoken of thermal runaway. To probe the trigger reactions in the batteries, partial cells were employed to simulate all the possible exothermic reactions in the battery.
Contribution of the exothermic reactions to thermal runaway
To detect the exothermic reactions of the concentrated LiFSI/DMC electrolyte in Gr|NMC811 bombardment, a comparison of the temperature dependence of dT/dt between the total jail cell and the partial cells is shown in Fig. 3. In contrast to the AnEly and CaAn partial cells, the CaEly partial cell did not enter thermal runaway. No precipitous temperature rise was observed from the ARC curve, and its T iii was 290 °C. Meanwhile, the maximum dT/dt was even lower than 0.i °C south−1, which is far lower than 1 °C s−1. These results illustrate that the estrus generated past the reactions inside the CaEly partial cell, including the cathode material decomposition and electrolyte oxidation by the cathode cloth, was relatively small before 290 °C and incapable of triggering the battery thermal delinquent. This consequence coincides with the depression flammability of the concentrated electrolyte, since the combustion reflected the intensity when the electrolyte was oxidized by the oxygen in the air, and the ARC result of the CaEly partial cell reflected the maximum intensity when the electrolyte was oxidized by the charged cathode or the oxygen released by the charged cathode. The AnEly and CaAn partial cells, on the reverse, could exist brought to thermal runaway (see Fig. 3). T two reached 200.5 °C, 202.five °C, and 225.1 °C in the example of the full cell, AnEly, and CaAn partial cells, respectively. In addition, the maximum (dT/dt)max for the full prison cell, AnEly, and CaAn partial cells was 401.ii °C due south−one, 882.9 °C due south−1, and 164.0 °C s−1, respectively. Thus, information technology tin be roughly concluded that both the AnEly and CaAn partial cells may contribute to thermal runaway in the full cell, but further analysis is needed to exactly observe out which of them provided the trigger reaction and which of them provided the main reaction during thermal delinquent.
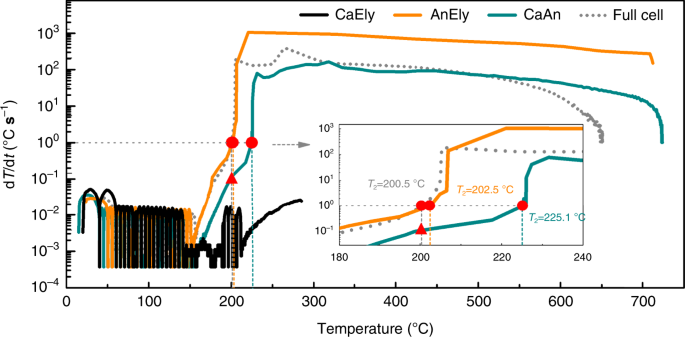
AnEly, CaEly, and CaAn fractional cells were prepared from the fully charged Gr|NMC811 batteries to investigate the contribution of different exothermic reactions during the thermal delinquent process of the battery. The temperature dependence of dT/dt betwixt the full cell (dot line in gray) and the partial cells was compared. No electrolyte in the CaAn jail cell, while the concentrated LiFSI/DMC electrolyte was used for all the other partial cells and the full battery.
To analyze the trigger reactions, the dT/dt values of all the cells at 200.5 °C (T 2 of the total cell) were compared. Information technology was observed that the dT/dt of the CaAn fractional prison cell was 0.1 °C s−1 (run into the triangle in Fig. 3) when the dT/dt of the full cell reached 1 °C due south−1 (see the circle in Fig. 3), meaning that the heat released by the CaAn partial cell at this temperature was no more than 1/10 of the total estrus of the full jail cell. In fact, the dT/dt of the CaAn partial cell was always ~1/10 of that of the full jail cell. Since the decomposition of the cathode material released picayune heat, the reactions inside the CaAn fractional jail cell contributed piffling to T 2 and the heat accumulation before T 2 for the full cell. Every bit a consequence, the CaAn partial prison cell cannot provide the trigger reaction. In dissimilarity, the dT/dt of the AnEly partial cell was close to i °C s−1 at T two, and the dT/dt curves of the AnEly and total cell nigh overlapped near T 2. This demonstrates that the heat release dynamics of the AnEly and full cell were almost the aforementioned. Thus, the chemical reactions between the anode-electrolyte were exactly the same reactions that took identify in the full cell. Since the battery was uniformly heated and the cell was so pocket-size that its thermal conductivity was quite good, the reactions would uniformly take place inside the cell. In addition, the exponential growth of the dT/dt with the temperature would be attributed to exothermic chemical reactions. That is, multiple reactions were involved between the anode and the electrolyte, and they were also initiated one after the other with a rise in temperature. In turn, the released heat led to a continuous increase in the temperature of the full prison cell. When the prison cell temperature became close to T ii, a group of vigorous reactions was initiated, enabling the total prison cell temperature surge at T 2. This chain reaction is very circuitous, and its analysis would be a bully take a chance for future research. Overall, the rise in the temperature curves of the AnEly and full cells was very like below T 2, which proves that the AnEly jail cell was responsible for the trigger reaction that led to the bombardment's thermal delinquent.
The surge after T ii could be obviously observed in the full, AnEly, and CaAn cells. Then, the dT/dt gradually decreased until the cell temperature reached the maximum value. Although the maximum temperature (T three) of the three kinds of cells is different, the maximum dT/dt of the AnEly and CaAn prison cell was both brought to hundreds of orders of magnitude, which indicates that the reactions in both the AnEly and CaAn cells were mainly responsible for the exothermic reactions during thermal delinquent. Too, T three of each of the AnEly and CaAn cells was higher than that of the total jail cell because some of the bombardment components did not exist in the AnEly and CaAn partial cells.
Q TR was used to announce the intensive heat release during thermal runaway, and it can be calculated from Eq. (i)13, where G denotes the mass of the jail cell (g), and C p signifies the specific heat capacity (J·g−one K−1; Supplementary Table 2 and Supplementary Note iv). The temperature range for the oestrus calculation is ΔT (ΔT =T three−T 1). Table one shows the characteristic temperatures and Q TR of the cells, and the equivalent rise in temperature of full bombardment (ΔT eq) which was caused by the reactions in the partial cells. ΔT eq was calculated from Eq. (2). The AnEly partial cell released a Q TR of 7.0 kJ with a ΔT eq of 312.half-dozen °C, whereas the full cell released a Q TR of 11.5 kJ with a ΔT of 516.9 °C. Meanwhile, the CaAn partial jail cell generated a Q TR of 11.three kJ with a ΔT eq of 507.nine °C, implying that the redox reactions between the anode–cathode can also generate tremendous estrus from T two to T three 12,13. The AnEly partial prison cell, among the three partial cells, was get-go brought to a thermal runaway. If the AnEly partial cell contributed all the heat to the full cell, the reaction in the CaAn prison cell would at least provide heat of iv.5 kJ to the total prison cell. It indicates that the reactions in both the AnEly and CaAn cells were the principal reactions during the thermal runaway. Besides, even if the cathode was completely inert nether all the temperatures, the heat released by the reactions between the anode and the electrolyte can bring the full battery to thermal runaway state (run across details in Supplementary Table 3 and Supplementary Note 5). Similarly, the full cell can be brought to thermal delinquent but at a higher temperature if the electrolyte stays totally inert.
$$Q_{{\mathrm{TR}}} = {\mathrm{{\Delta}}}T{\sum} ({M\cdot C_{\mathrm{p}}})$$
(i)
$${\mathrm{{\Delta}}}T_{{\mathrm{eq}}} = \frac{{Q_{{\mathrm{TR}}}}}{{{\sum} ({Thousand\cdot C_{\mathrm{p}}}) }}$$
(ii)
Based on the above analysis, iii conclusions can be drawn for the Gr|NMC811 thermal runaway. First, the reactions betwixt the cathode–anode contributed little to T two and the heat aggregating earlier T two, which coincides with the low flammability of the concentrated electrolyte. Second, the reactions in the AnEly partial cell were responsible for the heat accumulation below T 2, equally well as the trigger reaction of the thermal runaway. Tertiary, the reactions in both the AnEly and CaAn partial cells were the master reactions during the thermal delinquent. In the following department, the thermal stabilities of the individual materials and their mixtures are estimated to farther probe the trigger reactions and chief exothermic reactions during thermal delinquent.
Thermal stability of LiFSI/DMC in Gr|NMC811 battery
A DSC-TG-MS test was used to characterize the thermal stability of the cell components. Past enumerating all the thermal reactions of the individual and mixed prison cell components, the reactions inside the battery during the thermal runaway development can be screened out. Every bit the chemical reactions in the AnEly partial cell were targeted as the trigger reaction of thermal runaway. Then, starting time, all the possible reactions among the lithiated anode, full-bodied LiFSI/DMC electrolyte, electrolyte components, and delithiated cathode were measured (Fig. 4 and Supplementary Table iv).
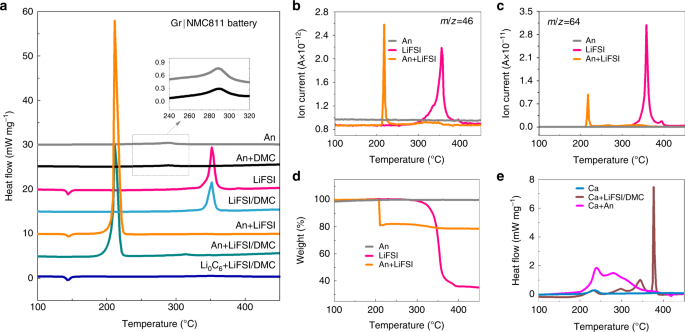
a DSC traces of the lithiated anode, concentrated LiFSI/DMC electrolyte components, and their mixtures for the Gr|NMC811 battery. The inset displays the enlarged peaks of An and An + DMC. b NOii (m/z = 46) gas development of LiFSI, the lithiated anode and their mixture during the DSC measurement. c And so2 (m/z = 64) gas evolution of LiFSI, the lithiated anode, and their mixture during the DSC measurement. d The weight loss of the lithiated anode, LiFSI, and their mixture. e DSC traces of the cathode, cathode mixed with concentrated LiFSI/DMC, and cathode mixed with anode.
Both the lithiated anode (An) and the An+DMC mixture exhibited a broad and balmy exothermic acme at ~289 °C (the dotted box and the inset in Fig. 4a), where the corresponding ΔH for both of them was ~70 J 1000−1. This exothermic pinnacle can be attributed to the reaction betwixt the lithiated graphite and the polyvinylidene fluoride-based binder12, which coincides with what was indicated past the plat TGA bend (Fig. 4d). In item, the weight was kept constant in the temperature range between the room temperature and 550 °C, which is consistent with the fact that no gases or volatilizable liquids were produced in the reactions between the lithiated graphite and the folder. The addition of DMC did not alter it, indicating that no reaction betwixt the An and DMC occurred. For LiFSI, the endothermic pinnacle at ~145 °C can be attributed to the melting of LiFSI, as no weight loss at this temperature occurred (Fig. 4d). As for the full-bodied electrolyte, the summit at ~145 °C disappears (LiFSI/DMC and An+LiFSI/DMC curves in Fig. 4a). The exothermic peak at ~350 °C might be associated with the thermal decomposition of FSI– 36,37, as 65% of the weight loss can be determined according to Fig. 4d, and the NO2 (m/z = 46) and so2 (m/z = 64) gases were released at ~350 °C because of the S–F and S–N breakage in the FSI− 36,37 (Fig. 4b, c). The concentrated electrolyte and LiFSI showed quite a similar exothermic peak at ~350 °C, which indicates the high thermal stability between LiFSI and DMC. Besides, the change from the crystal to the solution state did not change the decomposition beliefs of LiFSI.
Nevertheless, the addition of the lithiated anode results in obvious changes in the thermal behavior of LiFSI, which signifies the reaction betwixt them. As seen in Fig. 4a, for the mixture of LiFSI and An, when the lithiated anode was in contact with the concentrated LiFSI/DMC electrolyte, the sample showed a sharp exothermic peak (602.nine J yard−1) at 209.six °C, which was 8 times greater than that of the anode lone, while no exothermic peaks took identify at 350 °C. Similarly, for the An+LiFSI, NOtwo and Then2 gases were released at 210.9 °C, and they were accompanied by an intensive ΔH of 757.9 J chiliad−1. In add-on, the TGA curve (Fig. 4d) showed that a weight loss of nigh twenty% took place at ~210 °C, while the weight loss at ~350 °C was <three%, indicating that almost of the LiFSI powders reacted with the lithiated anode and produced gas or volatile products. It is reasonable that the DSC curve of the An+LiFSI/DMC sample did not show a LiFSI decomposition, equally the heat released past the 3% rest of the LiFSI was too little. Furthermore, for the fresh graphite (Li0C6), Li0Csix + LiFSI displayed an endothermic peak at 145 °C without the intensive exothermic behavior at ~210 °C, demonstrating the considerable heat produced in LiCvi + LiFSI from the chemical reaction betwixt intercalated lithium and LiFSI. It was also reported in ref. 38 that the battery employing the LiFSI-based electrolyte showed an exothermic peak of 1300 J g−one at ~200 °C, attributed to the chemical reduction of the FSI− anion by the lithiated anode38. For comparison, the thermal behaviors of LiPF6 and An+LiPFvi were also investigated, it demonstrated that LiPF6 was not involved in the trigger or main reactions of thermal runaway, coincided with the different T ii values of the concentrated and conventional electrolytes (run across details in Supplementary Fig. 3 and Supplementary Note 7).
As shown in Fig. 4e, the NMC811 cathode at the full accuse state (Ca) presented a small ΔH of 100.half-dozen J 1000−i at 235.1 °C, which can exist attributed to the phase transition. The thermal behavior of Ca+LiFSI/DMC implies that the NMC811 cathode barely reacts with the concentrated LiFSI/DMC electrolyte before 320 °C (come across details in Supplementary Note 6). The exothermal elevation at 350 °C and 380 °C in Ca+LiFSI/DMC may non be triggered in CaEly fractional cell as ARC system will enter into a cooling mode if the jail cell does not go to thermal runaway at a pre-set temperature of 290 °C. Thus, the exothermic reaction subsequently 320 °C contributed less to the thermal runaway which coincides with the thermal runaway behavior of the CaEly partial jail cell. Additionally, Ca+Separator was also investigated and with a minor heat generation (Supplementary Fig. 4 and Supplementary Note 8). The Ca+An sample exhibited two major exothermic peaks that were centered at 239.5 °C and 279.4 °C, respectively. The ΔH values of the two exothermic reactions were calculated, and they were found to be 834.0 J g−1 in full, where it was believed that they emanated from the consumption of the cathode-produced oxygen past the anode12. The reaction betwixt the cathode and the anode also significantly contributed to the heat during thermal runaway, but it was not the trigger reaction, which coincides with the Q TR analysis and thermal delinquent behavior of the CaAn partial cell.
Ii conclusions tin be fatigued from Fig. four. First, the LiFSI tin can exist reduced by the charged anode with gas evolution and the released intensive heat at ~210 °C, which was the trigger reaction that brought the battery to the bespeak of thermal runaway. 2d, the reaction betwixt the fully charged cathode and the anode generated bully rut, which significantly contributed to the released heat during thermal runaway, but information technology was not the trigger reaction.
Thermal stability of LiFSI/DMC in Gr|NMC532 battery
It is widely accepted that charged cathodes take part in the thermal runaway process and that the NMC532 battery is more than thermally and chemically stable than the NMC811 battery when the other battery materials are the aforementioned. According to research on the NMC811 battery with the LiFSI/DMC full-bodied electrolyte, the charged cathode only participated in the thermal runaway, while the trigger reaction was led by the anode and the concentrated electrolyte. As well, the Gr|NMC532 battery was farther used to confirm thermal runaway evolution when employing concentrated LiFSI/DMC electrolytes (Fig. v). Similar to the case of the Gr|NMC811 bombardment, the An and Ca individual samples resulted in a very weak exothermic peak, with ΔH values of 68.ii and 46.8 J chiliad−1, respectively (the insert in Fig. 5a and Supplementary Table 5), indicating that without a potent oxidizer or redactor, An and Ca cannot cause great damage by thermal decomposition. Equally for their mixtures, ii peaks appeared at 272.1 °C and 394.3 °C with a total ΔH of 709.three J g−i. This intensive heat was considered to bring the Gr|NMC532 bombardment with the conventional electrolyte to thermal runaway12. Still, for the concentrated electrolyte, the LiFSI exhibited an energetic tiptop of ~350 °C (Fig. 5a). In dissimilarity, the An+LiFSI mixture only showed one exothermic peak at 210.five °C with a ΔH of 767.8 J g−ane and with a simultaneous release of NO2 so2 gases (run into Fig. 5a–c). This alter indicates that reactions took identify between An and LiFSI. Similar to the results in the Gr|NMC811 battery, where the An induced the damage of the S–F and S–N bonds in the LiFSI with an intensive rut generation of ~210 °C, the heat was considerably high to crusade thermal runaway. Effigy 5d shows the TGA curves of the lithiated anode and LiFSI and their mixture. The DSC-TG-MS characteristics of the lithiated anode, electrolyte components, and their mixtures in the Gr|NMC532 battery coincided with the case of the Gr|NMC811 battery. Herein, for Gr|NMC532 bombardment with the concentrated LiFSI/DMC, the trigger reaction was besides demonstrated to exist LiC6 + LiFSI.
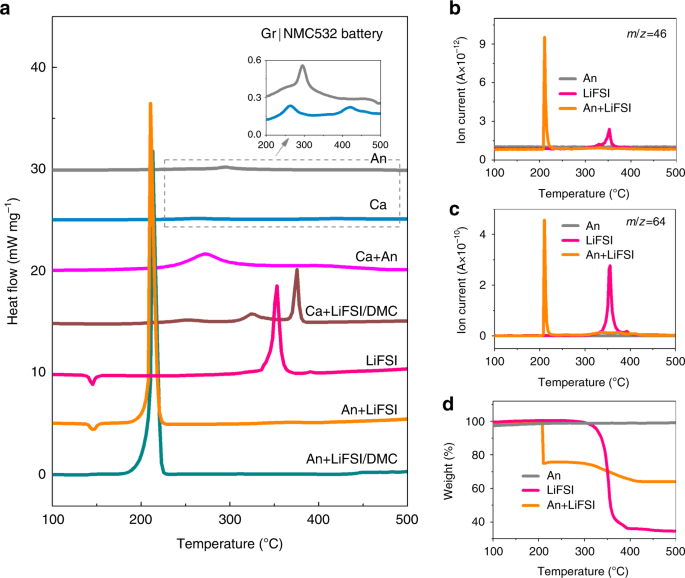
a DSC traces of the lithiated anode, cathode, concentrated LiFSI/DMC electrolyte components, and their mixtures for the Gr|NMC532 battery. The inset displays the enlarged peaks of Ca and An.b NOtwo (m/z = 46) gas evolution of the lithiated anode, LiFSI, and their mixture during the DSC measurement. c SO2 (m/z = 64) gas evolution of the lithiated anode, LiFSI, and their mixture during the DSC measurement. d The weight loss of the lithiated anode, LiFSI, and their mixture.
Non-flammable LiFSI/TMP in Gr|NMC811 battery
The release of DMC below T ii was an interference gene when analyzing the combustion of the Gr|NMC battery with the LiFSI/DMC concentrated electrolyte, equally the small-scale amount of free DMC is combustible though the flammability is greatly reduced. And then, the not-flammable concentrated LiFSI/TMP electrolyte was too examined in the Gr|NMC811 battery to further verify the vigorous reactions between the lithiated graphite and the LiFSI. Unfortunately, the battery was brought to thermal runaway at a T 2 of 195.2 °C (Fig. 6a), which is even less than the T 2 of the bombardment with the LiFSI/DMC full-bodied electrolyte. Equally shown in Fig. 6b, c, the An+LiFSI/TMP sample showed an intensive exothermic peak at ~210 °C, which was accompanied past a release of NOii and SO2 gases. The ΔH was found to exist 540.4 J g−1, which is lower than that of the LiFSI/DMC full-bodied electrolyte. However, the TMP could not prevent the exothermic reactions of LiCvi + LiFSI. As a result, the thermal runaway of the battery can still exist triggered and and then proceeded fifty-fifty with the non-flammable electrolyte. Meanwhile, a tearing flame could still be observed in the lateral heating test (the inset in Fig. 6a and Supplementary Movie 2). This indicates that, although the concentrated LiFSI/TMP electrolyte was non-flammable, the reactions betwixt the anode and the electrolyte, likewise equally the reactions between the cathode and the anode, were vigorous enough to cause a strong fire. It is known that combustion takes place every bit a result of the reaction betwixt the electrolyte and oxygen. Herein, for LiFSI-based full-bodied electrolytes, the trigger reaction was between the anode and the electrolyte (Fig. 6d), and the reaction that contributed to the thermal runaway was the redox reaction between the cathode–anode. Both reactions had zip to exercise with the flammability of the electrolytes. Thus, the battery safe cannot exist estimated based on the flammability of the used electrolytes. Overall, the complex reactions amongst the prison cell components should be carefully taken into consideration for battery safety assessments.

a The temperature dependence of dT/dt of the Gr|NMC811 battery with the concentrated LiFSI/TMP. The inset shows the combustibility of bombardment in the lateral heating examination. b DSC trace and TGA curve of the An+LiFSI/TMP sample. c NO2(chiliad/z = 46) and SOtwo(thou/z = 64) gas evolution of the An+LiFSI/TMP sample. d Illustration of the proposed thermal runaway mechanism of LiFSI-based full-bodied electrolytes in Gr|NMC batteries. The considerable heat generated by the reaction of LiFSI+LiChalf dozen triggers the Gr|NMC batteries to thermal runaway.
Mail service-examination assay
An XPS analysis was conducted on the DSC residual to further support the thermal delinquent mechanism. During the DSC measurement, the reaction of the LiFSI with the lithiated anode was ceased at 230 °C, which was at the finish of the exothermic peak (Supplementary Fig. 5a). After cooling to room temperature, the sample was transferred for the XPS assay (Supplementary Fig. 5b–eastward). The byproducts validated the chemical reactions betwixt the LiFSI and LiCsix during the thermal runaway, supposing that the tremendous heat was initiated by breaking the S-F and S-N bonds with the germination of LitwoCOiii, Li2SO3, Li2SOfour, LiF, and so on (see details in Supplementary Annotation 9). The XPS assay of An+LiFSI/TMP was also investigated (Supplementary Fig. six and Supplementary Annotation 10).
Discussion
This study revealed that batteries with LiFSI-based concentrated electrolytes also undergo thermal runaway. In the conducted experiments in this study, although the burn-retardant agent acted as a solvent, lithium salt acted every bit a potent oxidant, and the fire-retardant agent could not hinder the reactions betwixt the lithium salt and the lithiated anode. Likewise, the reactions between the anode and the cathode were constitute to significantly contribute to the rut output during thermal runaway, where the burn down-retardant agent could not work. The flammability of electrolytes does contribute to thermal runaway for the batteries with conventional flammable electrolytes, merely it is non the but contributable reaction and it is even non the biggest contributor to the strong cathode oxidization similar the NMC811. Thus, the interactions between the charged electrodes and electrolytes should be fully considered in bombardment prophylactic assessments. These findings provide valuable insights into the thermal delinquent mechanisms of concentrated electrolytes, including water-in-common salt aqueous electrolytes in LIBs.
Methods
Materials and batteries
The solvents of EC (ethylene carbonate) and EMC (ethyl methyl carbonate) and the electrolytes of LiFSI/DMC (1:ane.nine past molar), LiFSI/TMP (i:one.9 by tooth), and 1 M LiPFvi in EC:EMC (iii:7 by volume) were purchased from Dodochem Ltd. The moisture content of the solvents and electrolytes was <twenty ppm. 0.95 Ah Gr|NMC811 and 1.ii Ah Gr|NMC53 pouch cells with a ceramic coated PE separator were used in this work. The Gr|NMC811 and Gr|NMC532 dry cells were manufactured by Guangdong Canrd New Energy Technology Co., Ltd. The LiFSI/DMC and LiFSI/TMP concentrated electrolytes were injected into the dry cells with 3.6 mL per Ah, and the formation of all the batteries was performed at C/x nether 45 °C. The cells were cycled in the voltage range of 2.85–4.two Five at 1/3 C under 25 °C. The energy densities of the Gr|NMC811 and Gr|NMC532 batteries were 191 Wh kg−1 and 182 Wh kg−one, respectively (see details in Supplementary Table 6 and Supplementary Note 11). All the batteries were charged to 4.2 Five earlier the disassembly and measurements.
Bombardment safety evaluation
Bombardment safety was systematically assessed at the cell and textile levels in this study as shown in Fig. seven. In detail, the thermal properties of the concentrated electrolytes were characterized by an ignition exam, TGA and DSC. The prophylactic performance of the battery was evaluated by an ARC test. The combustibility of the battery was measured by lateral heating. DSC-TG-MS was used to probe the reactions during the thermal runaway evolution, revealing the thermal runaway machinery. Fractional cells were used to simulate the reactions between different battery components in the battery environment, and an ARC test was employed to tape the heat catamenia. The reactions between the unlike bombardment components were also investigated with DSC-TG-MS, and the results provided the reaction dynamics in the material level.
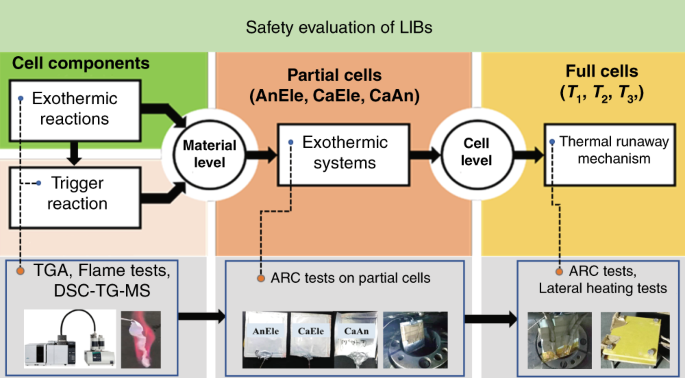
Characterization of battery safety at the prison cell and textile levels and the corresponding tests.
The associates of the partial cells is shown in Supplementary Fig. 7 and Supplementary Note 12, and the detailed description is presented in ref. eleven. Afterward removing the cathode from the fully charged Gr|NMC811 battery, the cell that just retains the lithiated anode and full-bodied LiFSI/DMC was sealed and named every bit the AnEly partial jail cell. Similarly, the CaEly partial cell was prepared. As for the CaAn fractional prison cell, the charged cathode and anode were done with DMC and naturally stale, and then the cathode and anode were rolled without a separator to gather the CaAn fractional cell33. All the higher up processing steps were performed in an argon-filled glove box in which the oxygen and water contents were controlled below 0.i ppm. The mass of active materials in AnEly, CaEly, and CaAn were exactly the same with the full prison cell, and these three kinds of partial cells represent the principal thermodynamic systems in the battery. The ARC exam was then practical to measure these partial cells so as to analyze the trigger reaction of the thermal runaway.
ARC exam protocol
A standard accelerated rate calorimetry (ARC-ES) musical instrument with an internal bore of 10 cm and a depth of 10 cm, which was manufactured by Thermal Chance Technology, was used to evaluate the thermal runaway features. The heat-wait-seek method was conducted on the ARC to detect the adiabatic cocky-heating rate as a function of time1,13. A heating step of 5 °C with a wait time of 15 min was performed on the ARC starting from 40 °C. The feature temperatures (T 1, T 2, and T 3) were extracted based on the data analysis. T 1 is defined as the cocky-heating temperature of the battery, where self-heating is identified when the battery's temperature increment rate reaches 0.02 °C min−1 without external oestrus. T 2 refers to the onset temperature of the thermal runaway, which is recorded when the battery'due south temperature rising rate (dT/dt) reaches one °C s−1. T 3 is the bombardment's highest temperature during the thermal runaway1,xiii. Notation that ARC system will enter into the cooling mode if the cell does not go to thermal delinquent at a pre-set temperature of 290 °C. Meanwhile, the existent-time open-excursion voltage (OCV) of the battery was recorded during the ARC test.
Before the ARC test, micro-thermocouples were inserted into the internal central positions of the tested partial and full cells as shown by the cerise arrows (Supplementary Fig. 7a). And so, the cells were resealed with a silicone sealant that can maintain tightness and elasticity even at 343 °C. The micro-thermocouples probed the real-time temperature of the cells during the ARC measurement.
Lateral heating test protocol
The combustibility of the Gr|NMC811 batteries with LiFSI/DMC (1:one.ix by molar) and LiFSI/TMP (1:one.9 by molar), respectively, was examined by a lateral heating test39. In detail, a ceramic heater, 28 mm in diameter, was fixed on the centre of the battery's surface. Then, the battery and the pad were clamped together with ii epoxy plates (Supplementary Fig. 7b). When the measurement started, the ceramic heater was connected with a xx West DC ability to heat the battery to 200 °C. The whole process was video-recorded, and the combustibility feature of the battery was analyzed.
DSC-TG-MS test protocol
A DSC-TG-MS exam was used to characterize the thermal properties and gaseous products of the cell components. The experiments were performed on NETZSCH STA449F5-QMS403D. The anode and cathode samples were acquired by disassembling a fully charged battery. The electrode was firstly done with DMC to remove the adsorbed electrolyte, and and so the cathode and anode powders were scratched from the electrodes in an argon-filled glove box so dried at 60 °C in the same glove box. All the samples were pressed in an aluminum crucible in the glove box and heated from 50 °C to 600 °C at a heating charge per unit of 10 °C min−1 in a highly pure flowing argon atmosphere. The examined individual cell components or their mixtures by the DSC-TG-MS examination are listed in Table two. These eleven samples covered the key exothermic reactions in the battery33. In the DSC results, the heat flow was normalized past the total weight of Ca+An+LiFSI/DMC, which was calculated based on the measured weight of the test sample and the corresponding weight ratio of the examination sample in Ca+An+LiFSI/DMC. The composing proportion of Ca+An+LiFSI/DMC is the same as that of the total cell. Past these means, the tiptop intensity implies the heating result on the bombardment. The rut generations (ΔH) normalized past both the individual component weight and total weight are shown in Supplementary Tables 4 and 5.
XPS analysis
To probe the byproducts of the reactions between the lithiated anode and the full-bodied electrolyte, the sample for XPS (Ten-ray photoelectron spectroscopy) assay was prepared as follows. First, a mixture of the total charged anode and certain amounts of the electrolyte was heated to 230 °C with a heating rate of x °C min−1. And then, the solid residues were cooled to room temperature in flowing argon, collected, and analyzed by XPS, which was collected with a K-Alpha + spectrometer using the Thermo Fisher Scientific Co. The binding free energy (BE) scale was calibrated with a C i due south peak at 284.eight eV.
Information availability
The data that support the findings of this report are available from the corresponding author upon reasonable request.
References
-
Feng, X. N. et al. Thermal runaway features of big format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 255, 294–301 (2014).
-
Wang, Q., Mao, B., Stoliarov, South. I. & Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 73, 95–131 (2019).
-
Wang, Q. S. et al. Thermal runaway caused fire and explosion of lithium ion bombardment. J. Power Sources 208, 210–224 (2012).
-
Yamada, Y., Wang, J. H., Ko, S., Watanabe, E. & Yamada, A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 4, 269–280 (2019).
-
Feng, X.Due north. et al. Fourth dimension sequence map for interpreting the thermal delinquent mechanism of lithium-ion batteries with LiNixCoyMnzO2 cathode. Front. Energy Res. 6, 126 (2018).
-
Han, Ten. et al. A review on the key bug of the lithium ion battery degradation among the whole life cycle. eTransportation 1, 100005 (2019).
-
Ren, D. et al. A comparative investigation of aging furnishings on thermal runaway behavior of lithium-ion batteries. eTransportation two, 100034 (2019).
-
Zhang, Y., Wang, H., Li, Westward. & Li, C. Quantitative identification of emissions from abused prismatic Ni-rich lithium-ion batteries. eTransportation 2, 100031 (2019).
-
Gauthier, R., Hall, D. South., Taskovic, T. & Dahn, J. R. A joint DFT and experimental report of an imidazolidinone additive in lithium-ion cells. J. Electrochem. Soc. 166, A3707–A3715 (2019).
-
Feng, 10. N. et al. Characterization of penetration induced thermal runaway propagation procedure within a large format lithium ion battery module. J. Ability Sources 275, 261–273 (2015).
-
Li, Y. et al. Thermal runaway triggered past plated lithium on the anode after fast charging. ACS Appl. Mater. Interfaces eleven, 46839–46850 (2019).
-
Liu, X. et al. Thermal runaway of lithium-ion batteries without internal curt circuit. Joule two, 2047–2064 (2018).
-
Feng, Ten. Due north. et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal assay database. Appl. Energ. 246, 53–64 (2019).
-
Tanim, T.R. et al. Avant-garde diagnostics to evaluate heterogeneity in lithium-ion battery modules. eTransportation three, 100045 (2020).
-
Tomaszewska, A. et al. Lithium-ion bombardment fast charging: a review. eTransportation 1, 100011 (2019).
-
Tian, J., Xiong, R. & Shen, W. A review on state of health interpretation for lithium ion batteries in photovoltaic systems. eTransportation two, 100028 (2019).
-
Yan, P. et al. Coupling of electrochemically triggered thermal and mechanical furnishings to aggravate failure in a layered cathode. Nat. Commun. ix, 2437 (2018).
-
Manthiram, A. A reflection on lithium-ion bombardment cathode chemistry. Nat. Commun. 11, 1550 (2020).
-
Zhu, Y. et al. Fast lithium growth and curt circuit induced by localized-temperature hotspots in lithium batteries. Nat. Commun. 10, 2067 (2019).
-
Wang, J. et al. Fire-extinguishing organic electrolytes for safe batteries. Nat. Free energy three, 22–29 (2017).
-
Pham, H. Q., Lee, H.-Y., Hwang, East.-H., Kwon, Y.-Thousand. & Vocal, Due south.-Due west. Not-flammable organic liquid electrolyte for loftier-condom and high-energy density Li-ion batteries. J. Power Sources 404, xiii–xix (2018).
-
Shi, P. et al. A highly concentrated phosphate-based electrolyte for high-rubber rechargeable lithium batteries. Chem. Commun. 54, 4453–4456 (2018).
-
Suo, L., Hu, Y. S., Li, H., Armand, M. & Chen, L. A new class of Solvent-in-Common salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. four, 1481 (2013).
-
Zeng, Z. et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries. Nat. Energy 3, 674–681 (2018).
-
Xiao, L. F. et al. Stable Li metal anode with "ion-solvent-coordinated" nonflammable electrolyte for safe Li metal batteries. ACS Energy Lett. 4, 483–488 (2019).
-
Piao, N. et al. Countersolvent electrolytes for lithium-metal batteries. Adv. Free energy Mater. x, 1903568 (2020).
-
Li, Thou., Wang, C., Chen, Z., Xu, Thousand. & Lu, J. New concepts in electrolytes. Chem. Rev. 120, 6783–6819 (2020).
-
Yamada, Y. & Yamada, A. Review—superconcentrated electrolytes for lithium batteries. J. Electrochem. Soc. 162, A2406–A2423 (2015).
-
Wang, J. et al. Superconcentrated electrolytes for a high-voltage lithium-ion bombardment. Nat. Commun. 7, 12032 (2016).
-
Shiga, T., Kato, Y., Kondo, H. & Okuda, C.-A. Self-extinguishing electrolytes using fluorinated alkyl phosphates for lithium batteries. J. Mater. Chem. A 5, 5156–5162 (2017).
-
Said, A. O., Lee, C., Stoliarov, South. I. & Marshall, A. W. Comprehensive assay of dynamics and hazards associated with cascading failure in 18650 lithium ion cell arrays. Appl. Free energy 248, 415–428 (2019).
-
Said, A. O., Lee, C., Liu, Ten., Wu, Z. B. & Stoliarov, S. I. Simultaneous measurement of multiple thermal hazards associated with a failure of prismatic lithium ion battery. Proc. Combust. Inst. 37, 4173–4180 (2019).
-
Ren, D. Southward. et al. Model-based thermal delinquent prediction of lithium-ion batteries from kinetics assay of cell components. Appl. Energy 228, 633–644 (2018).
-
Ren, D. S. et al. An electrochemical-thermal coupled overcharge-to-thermal-delinquent model for lithium ion bombardment. J. Power Sources 364, 328–340 (2017).
-
Gong, J., Wang, Q. & Lord's day, J. Thermal assay of nickel cobalt lithium manganese with varying nickel content used for lithium ion batteries. Thermochim. Acta 655, 176–180 (2017).
-
Eshetu, 1000. G. et al. Fire beliefs of carbonates-based electrolytes used in Li-ion rechargeable batteries with a focus on the role of the LiPF6 and LiFSI salts. J. Power Sources 269, 804–811 (2014).
-
Han, H.-B. et al. Lithium bis(fluorosulfonyl)imide (LiFSI) every bit conducting common salt for nonaqueous liquid electrolytes for lithium-ion batteries: physicochemical and electrochemical backdrop. J. Power Sources 196, 3623–3632 (2011).
-
Eshetu, Chiliad. Grand. et al. LiFSI vs. LiPF6 electrolytes in contact with lithiated graphite: Comparing thermal stabilities and identification of specific SEI-reinforcing additives. Electrochim. Acta 102, 133–141 (2013).
-
Gao, S. et al. Experimental written report on module-to-module thermal runaway-propagation in a battery pack. J. Electrochem. Soc. 166, A2065–A2073 (2019).
Acknowledgements
This work is funded by Ministry of Science and Technology of Red china (Grant No. 2019YFE0100200), National Natural Scientific discipline Foundation of China (Grant No. 51706117), China Postdoctoral Innovation Foundation (Grant No. BX20190162), Red china Postdoctoral Science Foundation (Grant No. 2019M660631), and the Tsinghua University Initiative Scientific Enquiry Program (Grant No. 2019Z02UTY06).
Writer data
Authors and Affiliations
Contributions
Thou.O. supervised this project. J.H. and X.N.F. conceived the original concept. J.H. and L.Westward. analyzed the data and wrote the paper. L.50., Y.Due north., A.O., 10.M.H., X.B.H., and I.O. revised the manuscript and triggered helpful discussion. D.R. helped J.H to deport the ARC tests and data analysis. Y.L., Due west.R., and Y.L.Fifty. helped J.H. to conduct DSC-TG-MS and XPS tests. All authors contributed to hash out the results.
Respective authors
Ideals declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Partha Mukherjee, Stanislav I. Stoliarov and the other, bearding, reviewer(s) for their contribution to the peer review of this piece of work.
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits utilise, sharing, accommodation, distribution and reproduction in whatsoever medium or format, as long every bit y'all give appropriate credit to the original author(s) and the source, provide a link to the Creative Eatables license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article'southward Creative Eatables license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a re-create of this license, visit http://creativecommons.org/licenses/by/iv.0/.
Reprints and Permissions
About this commodity
Cite this article
Hou, J., Lu, L., Wang, 50. et al. Thermal runaway of Lithium-ion batteries employing LiN(SO2F)2-based concentrated electrolytes. Nat Commun 11, 5100 (2020). https://doi.org/10.1038/s41467-020-18868-west
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/ten.1038/s41467-020-18868-w
Is Combustibility A Chemical Property,
Source: https://www.nature.com/articles/s41467-020-18868-w
Posted by: sancheznernat.blogspot.com


0 Response to "Is Combustibility A Chemical Property"
Post a Comment